The endocannabinoid system (ECS) was first described in the early 1990s. The discovery of compounds within the Cannabis sativa plant by Prof. Raphael Mechoulin’s group in the 1960s succeeded by pinpointing their likely targets, achieved by cloning specific cannabinoid receptors. Before that, researchers were unclear how cannabis produced its intoxicating or therapeutic effects. It was assumed that the high lipophilicity of cannabinoids accounted for their pharmacological action and that phytocannabinoids produced their effects by modifying the membranes of neurons in a fashion akin to many anaesthetics or by interfering with neural communication, as alcohol does.
Scientists ended up uncovering endogenous ligands for these cannabinoid receptors in the brain, referred to as endocannabinoids (eCBs). This significant development catalysed extensive advancements in understanding their physiological significance and the implications of cannabis and its derivatives in various health conditions, including mental well-being.
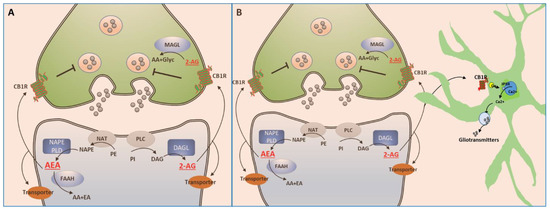
The ECS consists of the cannabinoid receptors, the endogenous cannabinoids (endocannabinoids or eCBs), the enzymes involved in the synthesis and degradation of eCBs and the protein(s) involved in the reuptake of secreted eCBs.
The ECS is not “new” or unique to humans, being present in most animals, from vertebrates to invertebrates, indicating great evolutionary value. It is present in the central nervous system, including the brain, and the peripheral nervous system, including organs, tissues, and immune cells. Its primary purpose is maintaining homeostasis in various bodily systems.
The ECS components
Endocannabinoids are lipidic compounds produced by the body that bind to cannabinoid receptors present in cells. The main endocannabinoids are N-arachidonoylethanolamine (AEA or anandamide) and 2-arachidonoylglycerol (2-AG). Other endogenous molecules with the capacity to modulate CB1/CB2 receptors have been discovered, including 2-AG ether, N-arachidonoyl-dopamine, O-arachidonoyl ethanolamine, and oleamide. However, the precise physiological roles of these latter substances have yet to be fully established.
Cannabinoid receptors belong to the G protein-coupled receptors family. The two main ones are CB1, found mainly in the brain and central nervous system, and CB2, found primarily in immune system cells and peripheral tissues. Endocannabinoids can bind not only to CB1 and CB2 receptors but also to other receptors, like TRPV1, involved in pain perception and also found in various tissues; PPARs (Peroxisome Proliferator-Activated Receptors) where they can impact metabolism, inflammation, and skin health; and GPR55, influencing developmental processes, pain, and inflammation. The transportation of endocannabinoids across the plasma membrane and their intracellular trafficking are still active research areas.
Enzymes Responsible for the Synthesis and Degradation of eCBs. AEA and 2-AG are synthesised when needed, mediating the suppression of neurotransmitter release by inhibiting voltage-gated calcium channels, activating K+ channels, inhibiting adenylate cyclase, and the cAMP/PKA pathway. The downstream effects depend on whether the neurotransmitter is excitatory or inhibitory. Importantly, recent research has uncovered intracellular reservoirs of endocannabinoids, challenging the classic understanding that they are strictly synthesised on demand. Once endocannabinoids have served their purpose, they are rapidly taken up by cells, metabolised, and degraded. Fatty acid amide hydrolase (FAAH) plays a crucial role in AEA degradation, while 2-AG degradation involves multiple enzymes, including MAGL. Interestingly, some of the degradation products of endocannabinoids have their own biological activities, contributing to ECS modulation and maintaining balance.
Location and function of eCBs in the body
CB1 receptors are primarily found in the central nervous system, including the brain and spinal cord. They are also found in peripheral tissues, including the liver, adipose tissue, and pancreas. CB1 receptors are the most abundant G protein-coupled receptors in the mammalian brain, and they are responsible for the psychoactive effects of cannabis.
Central nervous system: Mainly present, influencing emotions, cognition, memory, etc.
Cerebromicrovascular Endothelial Cells: Present, influencing vasodilation and inflammatory responses.
Peripheral Nervous System: Present, regulating nociception and sensory signalling.
Adipocytes: Activation can affect metabolism, insulin resistance, and depressive-like behaviour.
Liver: Present in low levels, upregulated in specific hepatic cells, contributing to insulin resistance and fibrosis.
Gut: Influence gastrointestinal functions, including motility and fluid secretion. The gut microbiota signal partly through the endocannabinoid network, which could be a potential source of information for new endocannabinoid-based therapies.
CB2 receptors, on the other hand, are predominantly expressed in immune tissues and cells but are also present at low levels in neuronal and non-neuronal cells of the brain. They are considered a potential target for specific and safe therapeutic drugs for inflammatory and autoimmune diseases, liver and kidney fibrosis, and neurodegenerative, neuroinflammatory, and neuropsychiatric disorders. However, the involvement of CB2 in neuropsychiatric disorders is still controversial, and more research is needed to determine its role in these conditions.
Central nervous system: Present, especially during inflammation.
Peripheral Nervous System: Predominantly found in immune cells.
Peripheral Tissues: Present in low levels, upregulated during inflammation.
Skin: Present and may affect sensory perception and skin health.
The endocannabinoid system is implicated in numerous physiological and pathological processes. How do plant cannabinoids interact with the ECS?
THC binds to CB1 and CB2 receptors and produces its psychoactive effects by activating CB1 receptors in peripheral and central nervous system cells, including but not exclusively neurons, which might be beneficial in neuropathic and inflammatory pain, neuropsychiatric disorders, neurological diseases, and inflammatory bowel disorders.
CBD does not directly bind to CB1 or CB2 receptors but has been shown to have a low affinity for both receptors. Instead, CBD interacts with multiple receptors and ion channels, including 5-HT1A, TRPV1, GPR55, and PPARγ, and it has been suggested to act as a negative allosteric modulator of CB1 receptors. CBD has been found to have potential as an antidepressant and anxiolytic agent, but more research is needed to determine its safety and efficacy in treating depression.
CBG has been found to have potential therapeutic effects in treating various conditions, including inflammation, pain, and cancer, by interacting with multiple receptors and ion channels, including CB1 and CB2 receptors, TRPV1, and 5-HT1A receptors.
CBN has a low affinity for both CB1 and CB2 receptors, but it has been found to have weak agonist activity at CB2 receptors. Its main indication is promoting sleep.
THCV has a low affinity for CB1 and CB2 receptors but has been found to act as an antagonist at CB1 receptors. It is structurally similar to THC but has different effects. It is believed to have appetite-suppressing and anti-convulsant effects and may help treat obesity and epilepsy.
Endocannabinoid Deficiency Theory
The fundamental premise of the Endocannabinoid Deficiency Theory revolves around the concept that all individuals possess an intrinsic endocannabinoid tone. This tone reflects the levels of endocannabinoids, such as anandamide and 2-AG, their production, metabolism, and the relative abundance and status of cannabinoid receptors within the body. According to this theory, the endocannabinoid tone can diminish under specific circumstances, whether congenital or acquired, leading to the emergence of pathophysiological syndromes.
Initially proposed in 2001 by Ethan Russo and subsequently developed, this theory found its basis in several factors. These included genetic overlaps and comorbidities, patterns of symptomatology that appeared to be influenced by the endocannabinoid system (ECS), and the observable symptomatic relief provided by exogenous cannabinoid treatments. Nevertheless, the theory lacked definitive objective evidence and formal clinical trial data.
In more recent times, however, significant strides have been made in substantiating the Endocannabinoid Deficiency Theory:
Statistically significant variations in cerebrospinal fluid levels of anandamide have been documented in individuals suffering from migraines. This finding suggests a potential link between endocannabinoid deficiencies and certain neurological conditions.
Advanced imaging studies have provided compelling evidence of ECS hypofunction in individuals diagnosed with post-traumatic stress disorder (PTSD). That indicates that disruptions in the endocannabinoid system may contribute to the pathophysiology of psychiatric disorders.
Ongoing clinical research has yielded data demonstrating a range of benefits associated with cannabinoid treatments. These benefits encompass alleviation of pain, improvement in sleep quality, and various other positive outcomes resulting from cannabinoid therapies and complementary lifestyle approaches targeting the ECS.
The Endocannabinoid Deficiency Theory continues to evolve, with accumulating scientific support suggesting that imbalances in the endocannabinoid system may play a pivotal role in developing and progressing various medical conditions. Further research is essential to understand this theory’s implications comprehensively and explore the potential therapeutic interventions it may inspire.
References
- Ahmad S, Hill KP. Medical Marijuana – A Clinical Handbook. 1st ed. Published October 27, 2021. ISBN: 978-3-030-78559-8.
- Andre CM, Hausman JF, Guerriero G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front Plant Sci. 2016;7:19. doi:10.3389/fpls.2016.00019.
- Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12(4):825-836. doi:10.1007/s13311-015-0387-1.
- Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol. 2010;159(1):129-141. doi:10.1111/j.1476-5381.2009.00515.x.
- Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9-29. doi:10.1038/s41582-019-0284-z.
- De Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, Machado S, Arias-Carrión O, Crippa JA, Silva AC. Antidepressant-like and anxiolytic-like effects of cannabidiol: A chemical compound of Cannabis sativa. CNS Neurolog Disord Drug Targets. 2014;13(6):953-960. doi:10.2174/1871527313666140612114838.
- Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov. 2018;17(9):623-639. doi:10.1038/nrd.2018.24.
- Hasbi A, Madras BK, George SR. Endocannabinoid System and Exogenous Cannabinoids in Depression and Anxiety: A Review. Brain Sciences. 2023;13(2):325. doi:10.3390/brainsci13020325.
- Hui-Chen Lu, Ken Mackie. Review of the Endocannabinoid System. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(6):607-615. doi:10.1016/j.bpsc.2020.07.016.
- Lowe H, Toyang N, Steele B, Bryant J, Ngwa W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int J Mol Sci. 2021;22(17):9472. doi:10.3390/ijms22179472.
- Mechoulam R. A Delightful Trip Along the Pathway of Cannabinoid and Endocannabinoid Chemistry and Pharmacology. Annu Rev Pharmacol Toxicol. 2023;63:1-13.
- Parker LA, Rock EM, Mechoulam R. CBD: What Does the Science Say? The MIT Press; 2022. ISBN: 978-1-9751-4189-9.
- Russo EB. Cannabis and Cannabinoid Research 2016, 1.1. http://online.liebertpub.com/doi/10.1089/can.2016.0009.
- Shahbazi, F., Grandi, V., Banerjee, A., & Trant, J. F. (2020). Cannabinoids and Cannabinoid Receptors: The Story so Far. Review, 23(7), 101301. doi:10.1016/j.isci.2020.101301
- Whiting ZM, Yin J, de la Harpe SM, Vernall AJ, Grimsey NL. Developing the Cannabinoid Receptor 2 (CB2) pharmacopoeia: past, present, and future. Trends Pharmacol Sci. 2022. doi:10.1016/j.tips.2022.06.010.
- Zou S, Kumar U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci. 2018;19(3):833. doi:10.3390/ijms19030833.

Made in California, with the finest ingredients, bcure products are made one by one with the highest quality control.

